INTRODUCTION
177Lu-PSMA-617 is a radiopharmaceutical that binds to the prostate-specific membrane antigen (PSMA) and delivers beta(-) radiation. Results from the Phase 3 VISION Trial demonstrated that 177Lu-PSMA-617 prolonged overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) who had received prior taxane-based chemotherapy compared with standard of care alone and paved the way for FDA approval of this therapy in March 2022.
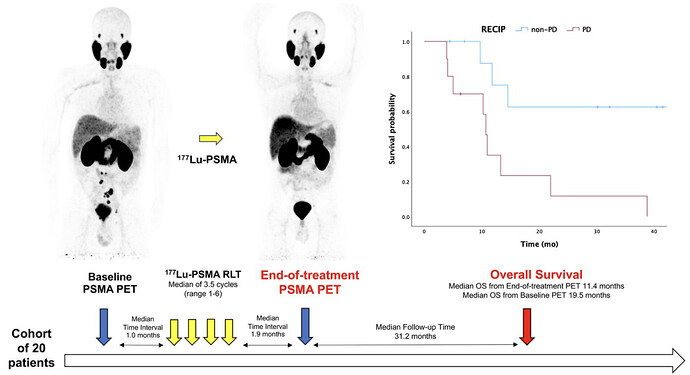
Prior studies have already evaluated the prognostic value of PSMA PET/CT in mCRPC patients treated with 177Lu-PSMA-617. Recently, Response Evaluation Criteria in PSMA-PET/CT (RECIP) 1.0 were introduced, and patients were classified as having progressive disease if they had at least a 20% increase in whole-body PSMA tumor volume with the appearance of new lesions on interim PSMA-PET after the first 2 cycles of 177Lu-PSMA-617. Progression on interim PSMA PET/CT by RECIP 1.0 was prognostic for OS in mCRPC patients treated with 177Lu-PSMA-617. In our retrospective, single-center study, we evaluated the prognostic value of end-of-treatment PSMA PET/CT (ePET) in this patient population.
RESULTS
20 mCRPC patients who underwent 177Lu-PSMA-617 with available baseline and ePET were included in our analysis. Patients with new lesions on ePET had shorter OS than those without new lesions (median OS, 10.7 mo [95% CI, 9.2-12.2] vs. not reached; P = 0.002). Patients with a greater than 20% increase in whole-body tumor volume had shorter OS than those who did not (median OS, 10.7 mo [95% CI: 9.7-11.7 mo] vs. not reached; P = 0.007). Patients with progressive RECIP also had shorter OS than those with non-progressive RECIP (median OS, 10.7 mo [95% CI, 9.7-11.7 mo] vs. not reached; P = 0.007).
Although RECIP 1.0 was initially introduced using interim PSMA PET/CT, our analysis now suggests that response assessment on ePET using RECIP 1.0 is prognostic for OS. Furthermore, while our analysis also shows that the appearance of new lesions on ePET was prognostic for OS, we observed that single lesion assessments may not fully capture disease heterogeneity. In our analysis, among 11 of 20 patients who had new lesions on ePET, 1 (9%) patient was classified as non-progressive by RECIP 1.0 and had an OS of 9.7 mo after ePET. In the original RECIP 1.0 study, which analyzed 124 patients, 13% of patients had new lesions despite a decrease in tumor volume and were classified as having stable disease, with a different survival outcome from true progressors. In a comparative analysis of criteria for therapy response assessment in mCRPC, RECIP 1.0 also identified fewer patients with progressive disease, and patients classified as having progressive disease had a higher risk of death than patients classified as having non-progressive disease by RECIP 1.0 compared with lesion-based assessments.
FUTURE DIRECTION
Larger prospective trials are necessary to define the prognostic value of progression on ePET by RECIP 1.0 for progression-free survival and OS. These trials could provide data to support the use of progression by ePET as a novel surrogate endpoint in clinical trials.